
Dosing and administration in AML1
In the treatment of AML, TIBSOVO® is given along with azacitidine. For the posology and method of administration of azacitidine, please refer to the Product Monograph for azacitidine.
Recommended dose and administration for TIBSOVO®
TIBSOVO® recommended dose
For the treatment of AML, the recommended dose is 500 mg TIBSOVO® (2 x 250 mg tablets) taken orally once daily.
TIBSOVO® method of administration
Do not eat anything for 2 hours before taking TIBSOVO® and until 1 hour after taking TIBSOVO®
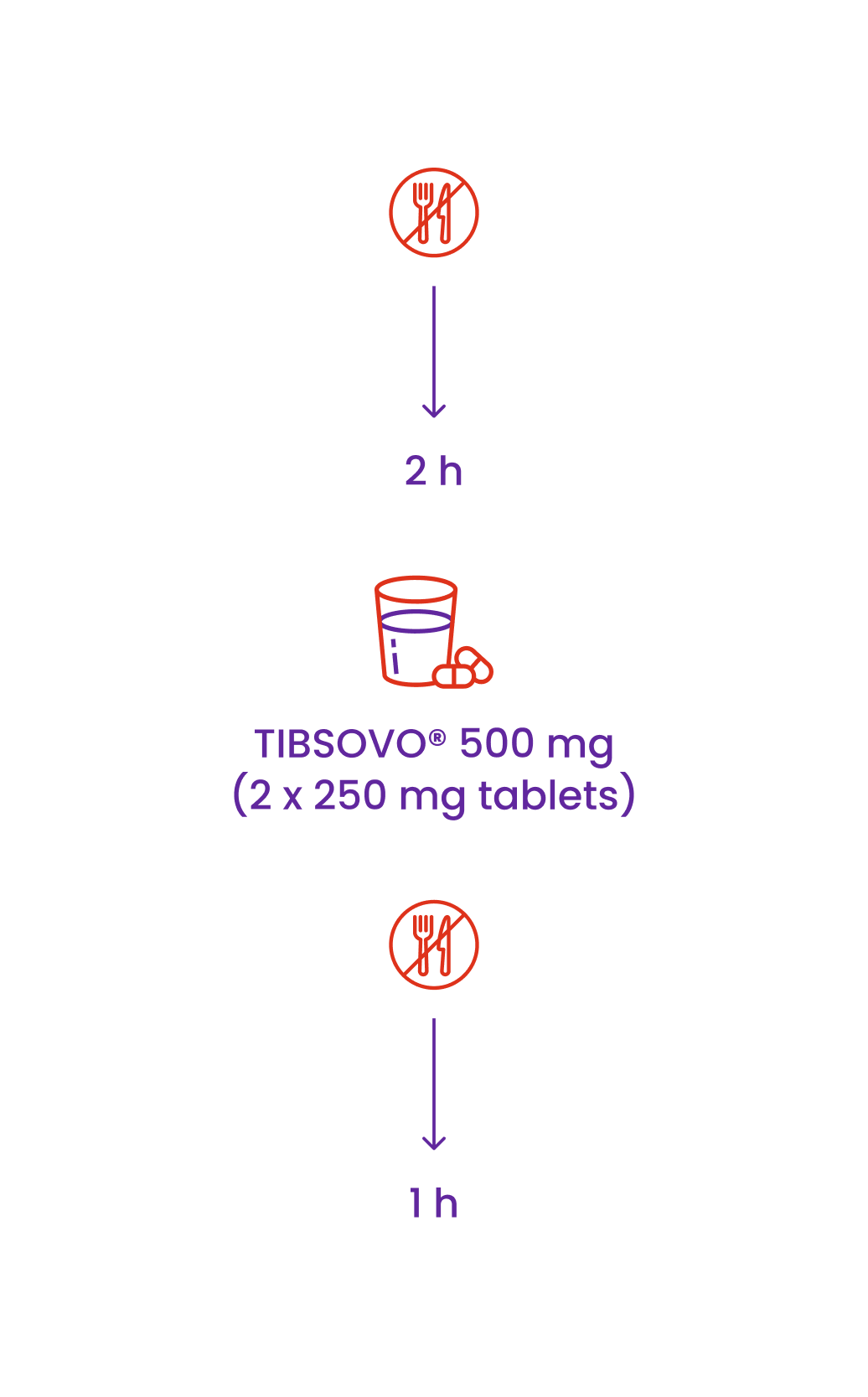
The tablets are taken at about the same time each day
The tablets should be swallowed whole with water
Grapefruit and grapefruit juice may increase plasma concentrations of TIBSOVO®.
Treatment should be continued until disease progression or until treatment is no longer tolerated.
Adapted from the TIBSOVO® Product Monograph. Please see the TIBSOVO® Product Monograph for complete dosing information.
TIBSOVO® 500 mg QD (approximately every 24 hours) orally
Adapted from the TIBSOVO® Product Monograph.
Please see the TIBSOVO® Product Monograph for complete dosing information.
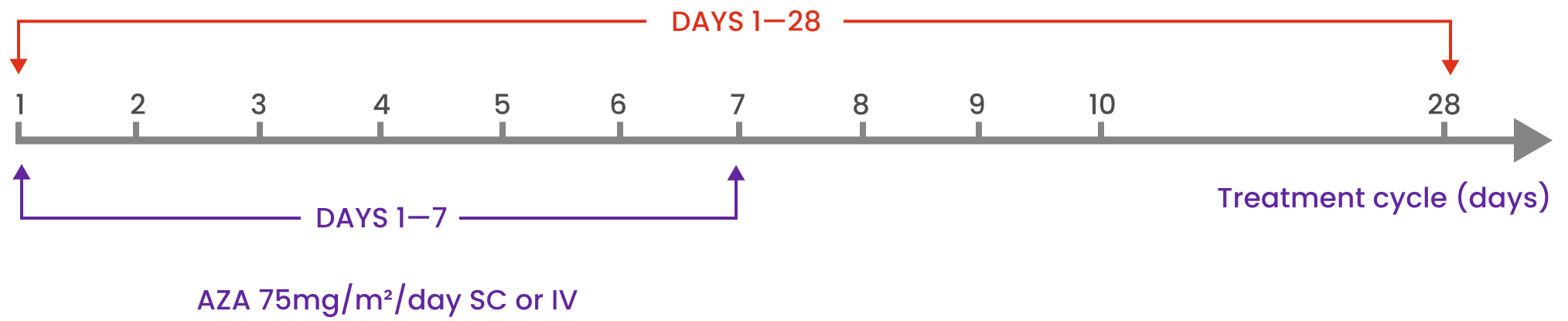
TIBSOVO® should be started on Cycle 1 Day 1 and administered once daily during the 28-day cycle. It should be started in combination with azacitidine at 75 mg/m2 of body surface area, intravenously or subcutaneously, once daily on Days 1-7 of each 28-day cycle. The first treatment cycle of azacitidine should be given at 100% of the dose.
It is recommended that patients be treated for a minimum of 6 cycles. Treatment should be continued until disease progression or until treatment is no longer tolerated by the patient.
Consult the TIBSOVO® Product Monograph
2-HG: 2-hydroxyglutarate; α-KG: alpha-ketoglutarate; AML: acute myeloid leukemia; AZA: azacitidine; PBO: placebo; CCA: cholangiocarcinoma; CI: confidence interval; CR: complete response; CRh: complete response with partial hematologic recovery; CTCAE: Common Terminology Criteria for Adverse Events; DLCO: diffusing capacity for carbon monoxide; ECG: electrocardiogram; ECOG PS: Eastern Cooperative Oncology Group Performance Status; EFS: event-free survival; eGFR: estimated glomerular filtration rate; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; IDH1: isocitrate dehydrogenase-1; IRC: Independent Radiology Centre; IV: intravenous; mIDH1: mutated isocitrate dehydrogenase-1; MOA: mechanism of action; NCCN: National Comprehensive Cancer Network; OR: odds ratio; OS: overall survival; PBO: placebo; PCR: polymerase chain reaction; PD: pharmacodynamics; PK: pharmacokinetics; QD: daily; RECIST: Response Evaluation Criteria In Solid Tumors; SC: subcutaneous.
References:
- TIBSOVO® Product Monograph. Servier Canada. July 19, 2024.
- Montesinos P, et al. N Engl J Med. 2022 Apr 21;386(16):1519–1531.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 2.2025 — January 27, 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Duct Cancers Version 6.2024 — January 10, 2025.
- Abou-Alfa GK, et al. Lancet Oncol. 2020 Jun;21(6):796-807.
- Zhu AX, et al. JAMA Oncol. 2021 Nov 1;7(11):1669-1677.
- Dammacco F, Silvestris F, eds. Oncogenomics: From Basic Research to Precision Medicine. Academic Press; 2019.
Stay Connected with Servier Canada
Stay up to date on disease information, treatment options, our products, and patient support programs.
