MOA, PD, and PK*
Mechanism of action1*
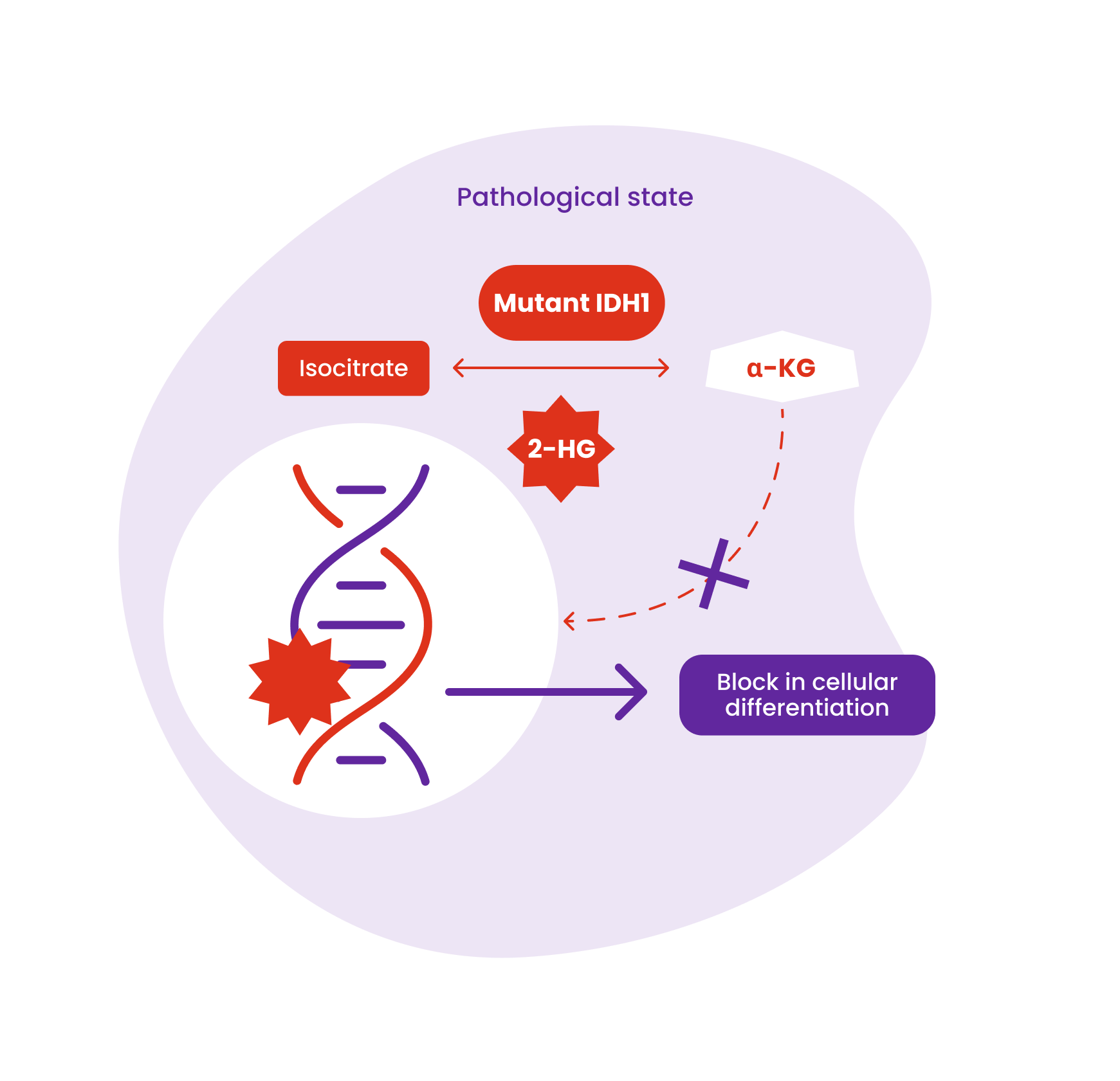
TIBSOVO® is an inhibitor of the mutant IDH1 enzyme
Mutant IDH1 converts alpha-ketoglutarate (α-KG) to 2-hydroxyglutarate (2-HG) which blocks cellular differentiation and promotes tumorigenesis in both hematologic and non-hematologic malignancies.
The mechanism of action of ivosidenib beyond its ability to reduce 2-HG and restore cellular differentiation is not fully understood across indications.
Adapted from the Product Monograph.
Pharmacodynamics*
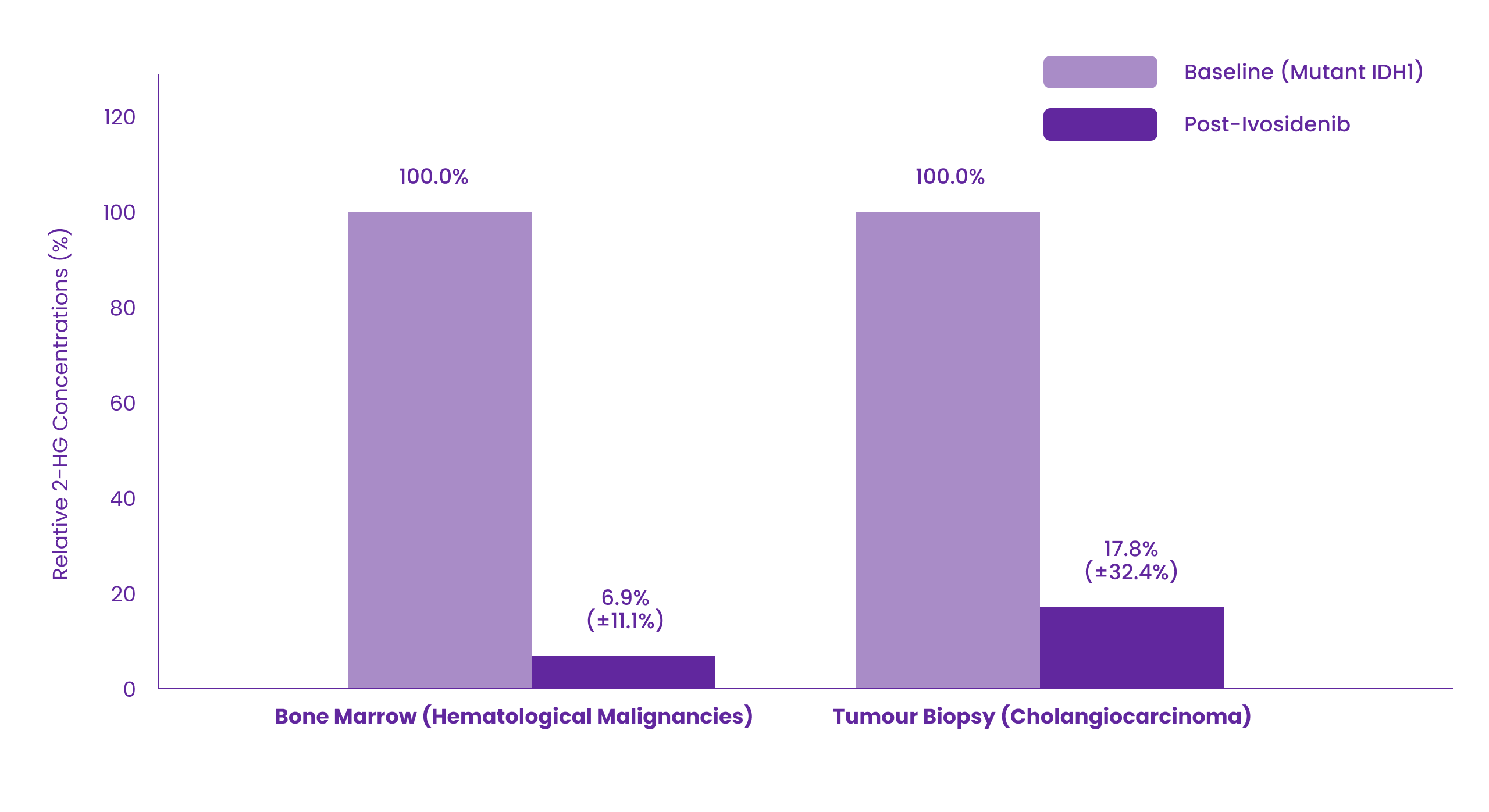
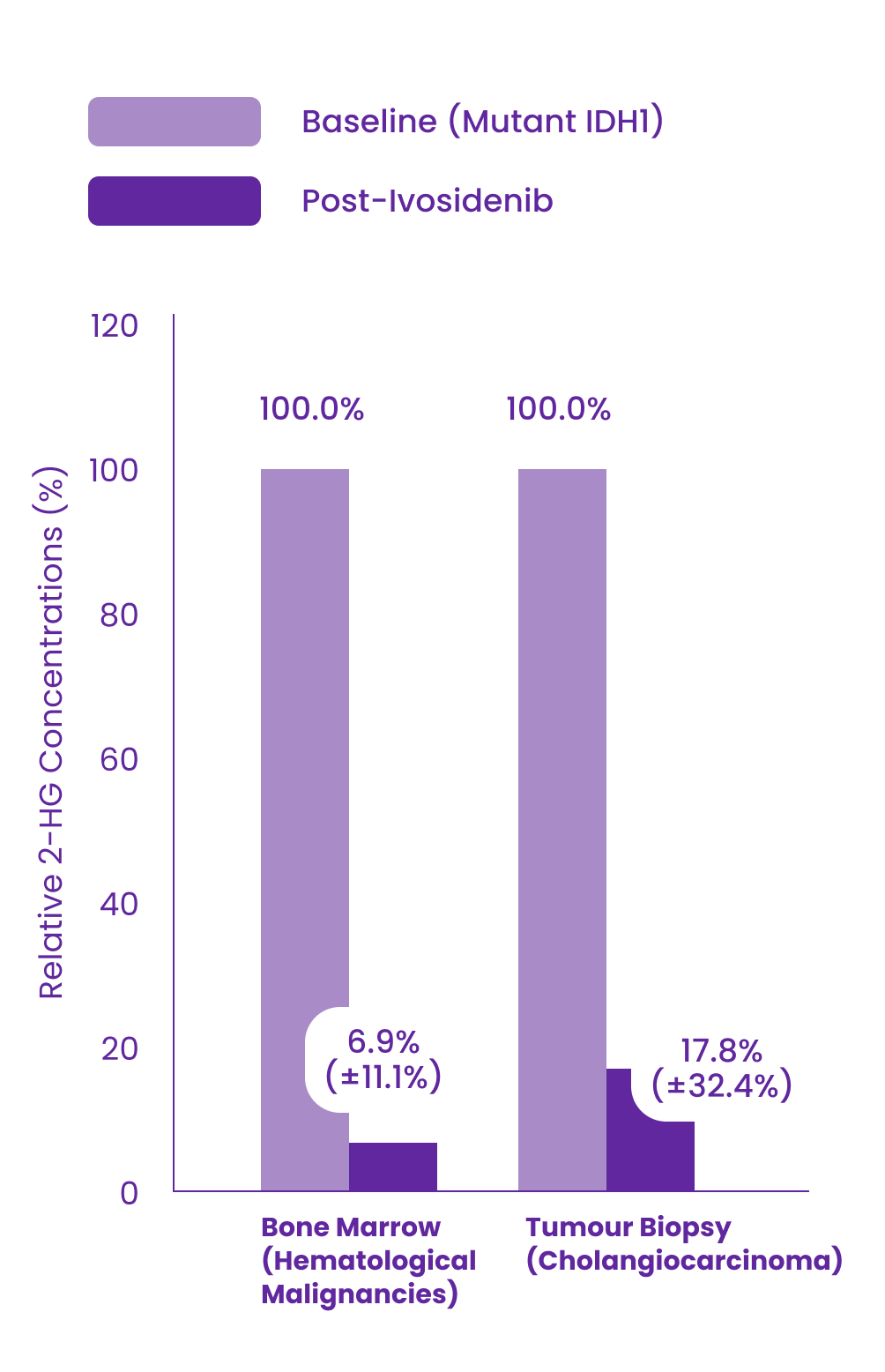
Multiple doses of ivosidenib 500 mg daily decreased plasma concentrations of 2-HG in patients with hematological malignancies and cholangiocarcinoma with mutated IDH1 to levels approximating those observed in healthy subjects. In bone marrow of patients with hematological malignancies and in tumour biopsy of patients with cholangiocarcinoma, the mean (% coefficient of variation) reductions in 2-HG concentrations were 93.1% (11.1%) and 82.2% (32.4%), respectively.
Adapted from the Product Monograph.
Pharmacokinetics in older patients*
No clinically meaningful effects on the pharmacokinetics of ivosidenib were observed in older patients up to 84 years. The pharmacokinetics of ivosidenib in patients 85 years of age or older is unknown.
*Clinical significance is unknown.
Consult the TIBSOVO® Product Monograph
2-HG: 2-hydroxyglutarate; α-KG: alpha-ketoglutarate; AML: acute myeloid leukemia; AZA: azacitidine; PBO: placebo; CCA: cholangiocarcinoma; CI: confidence interval; CR: complete response; CRh: complete response with partial hematologic recovery; CTCAE: Common Terminology Criteria for Adverse Events; DLCO: diffusing capacity for carbon monoxide; ECG: electrocardiogram; ECOG PS: Eastern Cooperative Oncology Group Performance Status; EFS: event-free survival; eGFR: estimated glomerular filtration rate; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; IDH1: isocitrate dehydrogenase-1; IRC: Independent Radiology Centre; IV: intravenous; mIDH1: mutated isocitrate dehydrogenase-1; MOA: mechanism of action; NCCN: National Comprehensive Cancer Network; OR: odds ratio; OS: overall survival; PBO: placebo; PCR: polymerase chain reaction; PD: pharmacodynamics; PK: pharmacokinetics; QD: daily; RECIST: Response Evaluation Criteria In Solid Tumors; SC: subcutaneous.
References:
- TIBSOVO® Product Monograph. Servier Canada. July 19, 2024.
- Montesinos P, et al. N Engl J Med. 2022 Apr 21;386(16):1519–1531.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 2.2025 — January 27, 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Duct Cancers Version 6.2024 — January 10, 2025.
- Abou-Alfa GK, et al. Lancet Oncol. 2020 Jun;21(6):796-807.
- Zhu AX, et al. JAMA Oncol. 2021 Nov 1;7(11):1669-1677.
- Dammacco F, Silvestris F, eds. Oncogenomics: From Basic Research to Precision Medicine. Academic Press; 2019.
Stay Connected with Servier Canada
Stay up to date on disease information, treatment options, our products, and patient support programs.