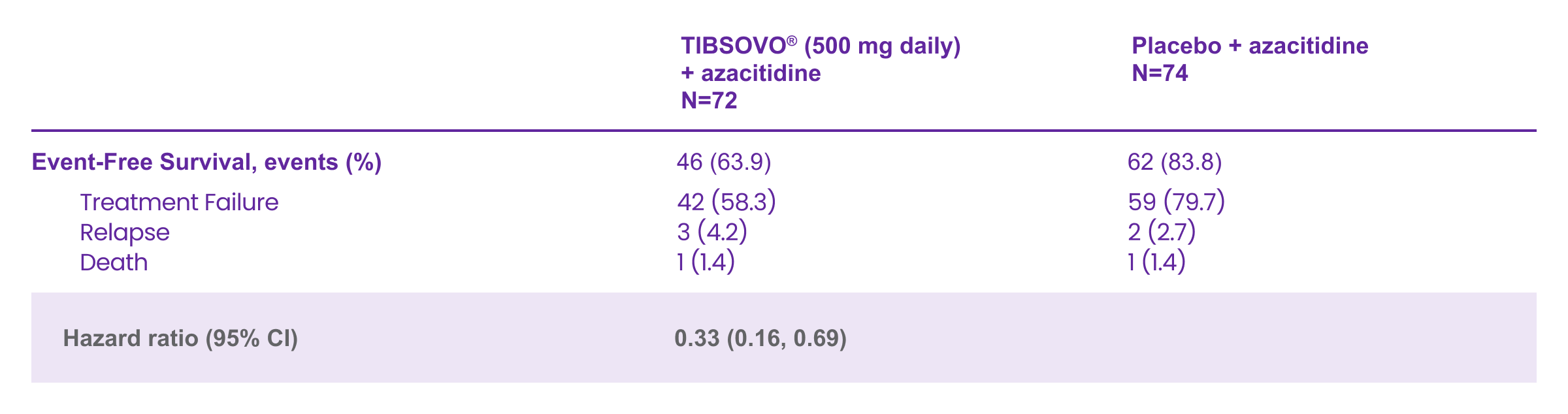
The AGILE study: Key efficacy results
Primary endpoint: TIBSOVO® + azacitidine delivered statistically significant improvement in event-free survival (EFS) compared with placebo + azacitidine1,2
Median follow-up: 12.4 months (range, 0.1-28.8).
Event-free survival (EFS) was measured from the date of randomization until treatment failure, relapse from remission, or death by any cause. Hazard ratio is estimated using a Cox’s proportional hazards model stratified by the randomization stratification factors (AML status and geographic region) with placebo + azacitidine as the denominator. 1-sided p-value < 0.0017 was required to achieve statistical significance.
Adapted from the TIBSOVO® Product Monograph
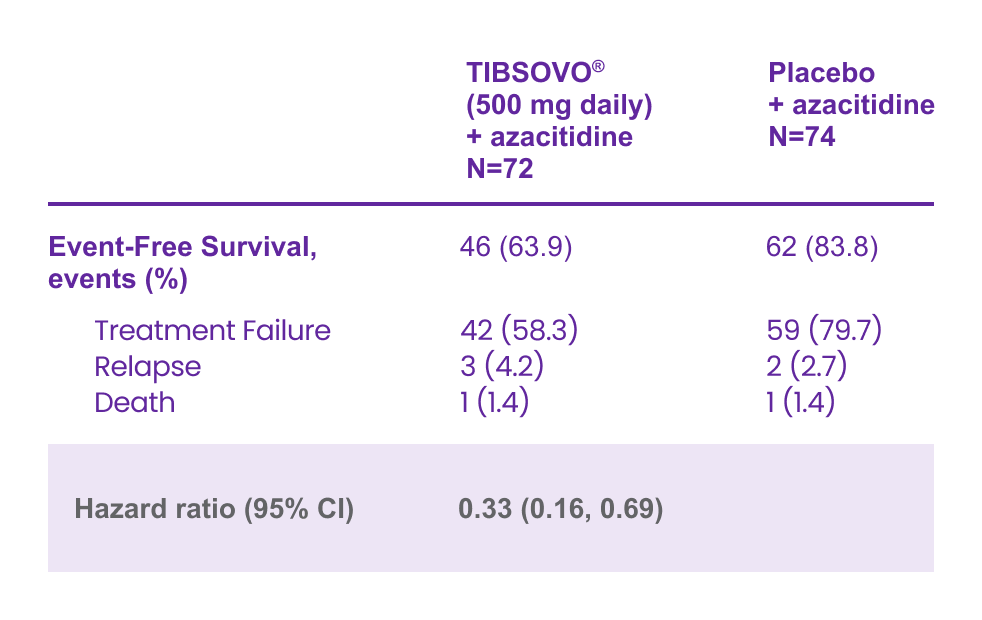
Key secondary endpoint: TIBSOVO® + azacitidine demonstrated an overall survival* benefit compared to placebo + azacitidine1,2
TIBSOVO® + AZA achieved 56% instantaneous risk reduction vs PBO + AZA
HR† 0.44 (95% CI, 0.27–0.73); p=0.001§
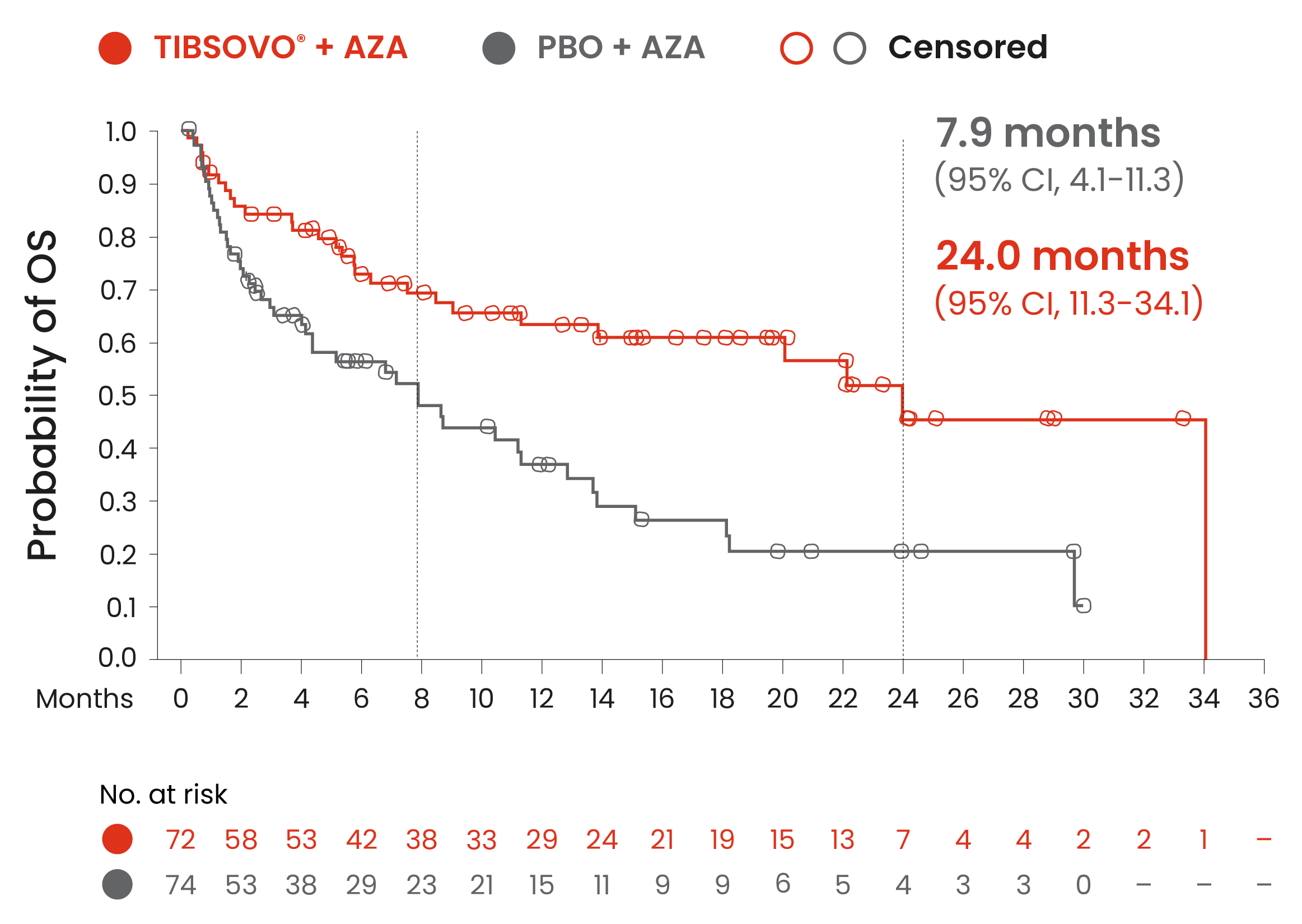
Median follow-up, 15.1 months (range: 0.2–34.1)
*OS was defined as the time from randomization to the date of death from any cause.
†HR is estimated using a Cox’s proportional hazards model stratified by the randomization stratification factors (AML status and geographic region) with placebo + azacitidine as the denominator.
§2-sided P-value.
Adapted from the TIBSOVO® Product Monograph and Montesinos et al. 2022.
An updated OS analysis, carried out at 64.2% (N = 95) of events, confirmed the overall survival benefit of TIBSOVO® in combination with azacitidine compared to placebo in combination with azacitidine with a median OS of 29.3 months vs 7.9 months, respectively (HR = 0.42; 95% CI: 0.27 to 0.65).1
Key secondary endpoint: TIBSOVO® + azacitidine demonstrated complete responses* compared to placebo + azacitidine1,2
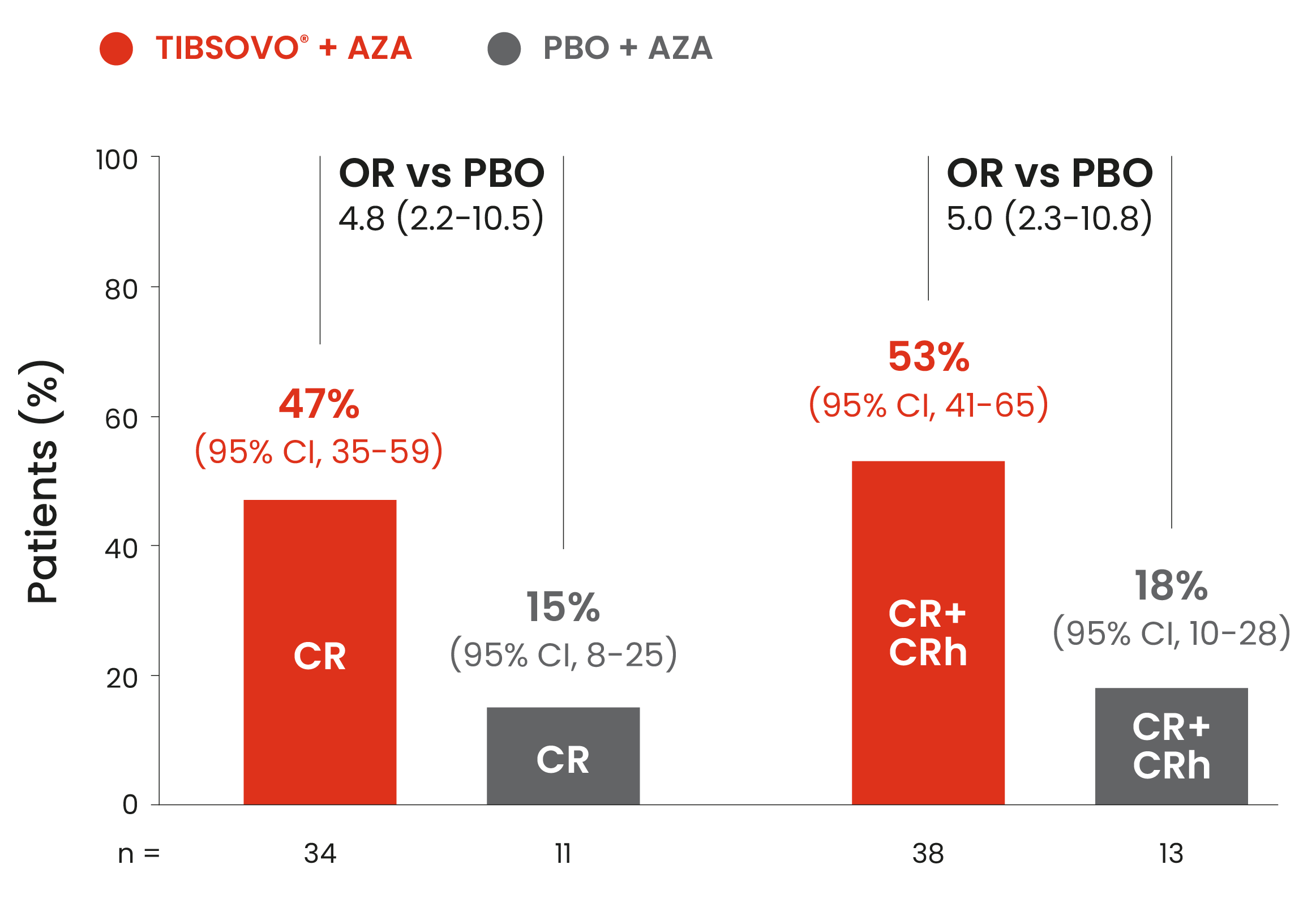
*Response was determined according to modified International Working Group criteria.
Adapted from the TIBSOVO® Product Monograph and Montesinos et al. 2022.
Consult the TIBSOVO® Product Monograph
2-HG: 2-hydroxyglutarate; α-KG: alpha-ketoglutarate; AML: acute myeloid leukemia; AZA: azacitidine; PBO: placebo; CCA: cholangiocarcinoma; CI: confidence interval; CR: complete response; CRh: complete response with partial hematologic recovery; CTCAE: Common Terminology Criteria for Adverse Events; DLCO: diffusing capacity for carbon monoxide; ECG: electrocardiogram; ECOG PS: Eastern Cooperative Oncology Group Performance Status; EFS: event-free survival; eGFR: estimated glomerular filtration rate; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; IDH1: isocitrate dehydrogenase-1; IRC: Independent Radiology Centre; IV: intravenous; mIDH1: mutated isocitrate dehydrogenase-1; MOA: mechanism of action; NCCN: National Comprehensive Cancer Network; OR: odds ratio; OS: overall survival; PBO: placebo; PCR: polymerase chain reaction; PD: pharmacodynamics; PK: pharmacokinetics; QD: daily; RECIST: Response Evaluation Criteria In Solid Tumors; SC: subcutaneous.
References:
- TIBSOVO® Product Monograph. Servier Canada. July 19, 2024.
- Montesinos P, et al. N Engl J Med. 2022 Apr 21;386(16):1519–1531.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 2.2025 — January 27, 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Duct Cancers Version 6.2024 — January 10, 2025.
- Abou-Alfa GK, et al. Lancet Oncol. 2020 Jun;21(6):796-807.
- Zhu AX, et al. JAMA Oncol. 2021 Nov 1;7(11):1669-1677.
- Dammacco F, Silvestris F, eds. Oncogenomics: From Basic Research to Precision Medicine. Academic Press; 2019.
Stay Connected with Servier Canada
Stay up to date on disease information, treatment options, our products, and patient support programs.