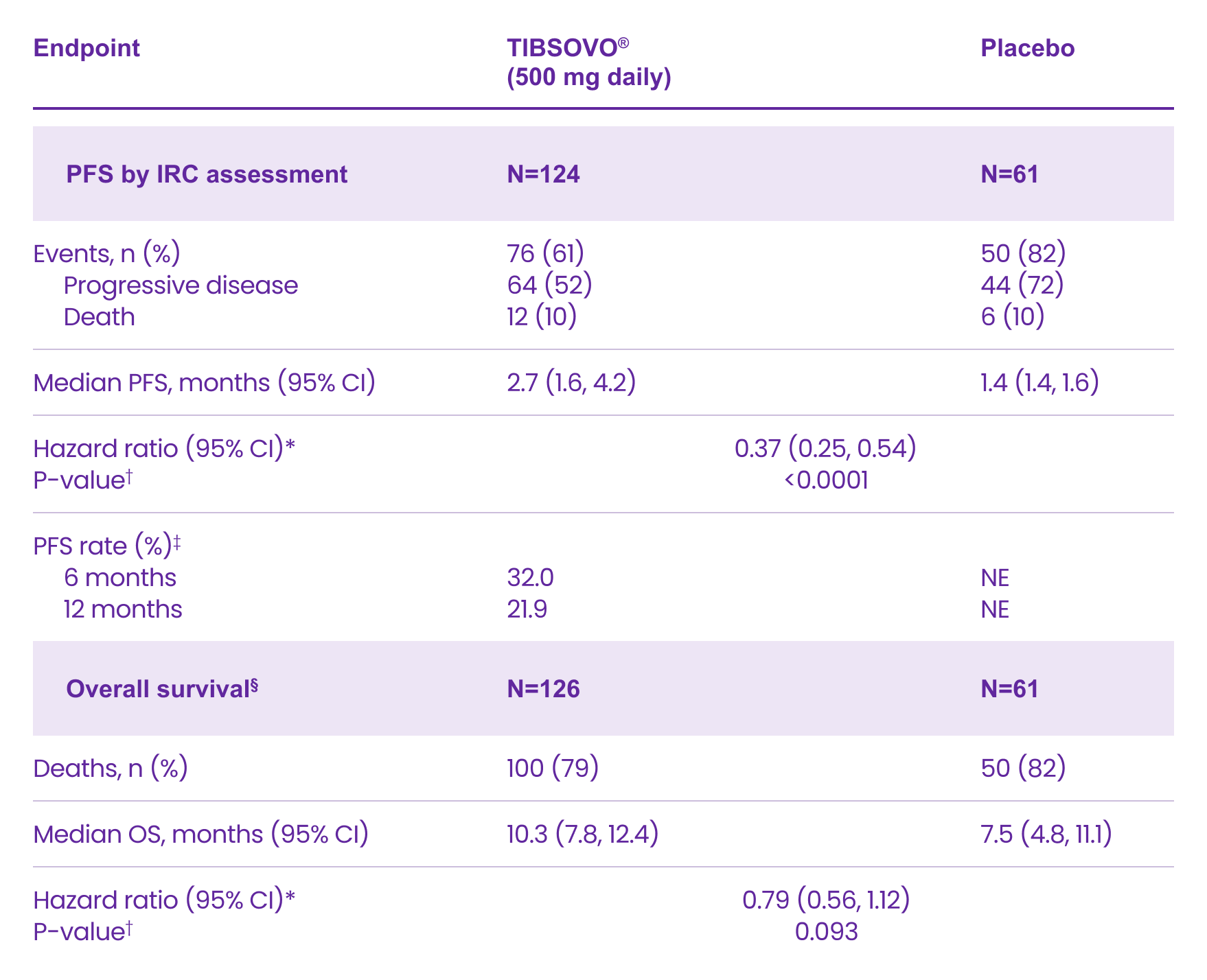
The CLARIDHY study: Key efficacy results1
Efficacy Results in Patients with Locally Advanced or Metastatic Cholangiocarcinoma
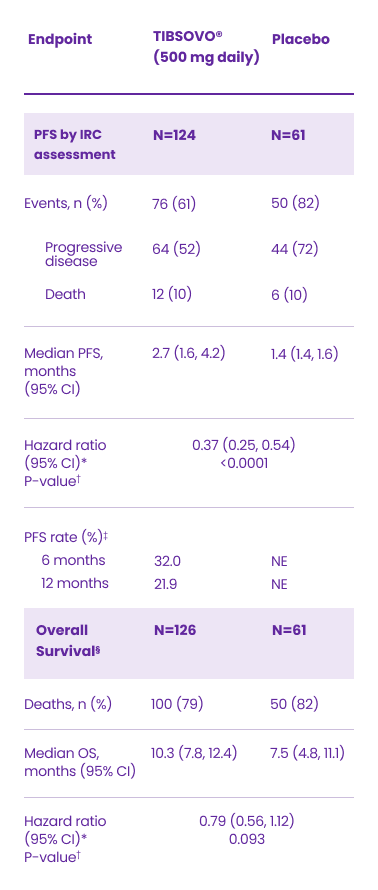
CI: confidence interval; IRC: Independent Radiology Centre; NE: not estimable; OS: overall survival; PFS: progression-free survival.
*Hazard ratios calculated from stratified Cox regression model. Stratification factor is the number of prior lines of therapy at randomization.
†P-value is calculated from the one-sided stratified log-rank test without adjusting for crossover. Stratification factor is the number of prior lines of therapy at randomization.
‡Based on Kaplan-Meier estimation. No patients randomized to placebo achieved PFS of 6 months or longer.
§OS results are based on the final analysis of OS (based on 150 deaths; data cut-off May 31, 2020) which occurred 16 months after the final analysis of PFS (data cut-off January 31, 2019).
Adapted from the TIBSOVO® Product Monograph.
TIBSOVO® demonstrated an improvement in PFS* compared to placebo
TIBSOVO® significantly reduced the instantaneous relative risk of progression or death by 63% vs placebo
HR 0.37; 95% CI, 0.25-0.54; 1-sided p<0.0001
Kaplan-Meier plot of PFS per IRC
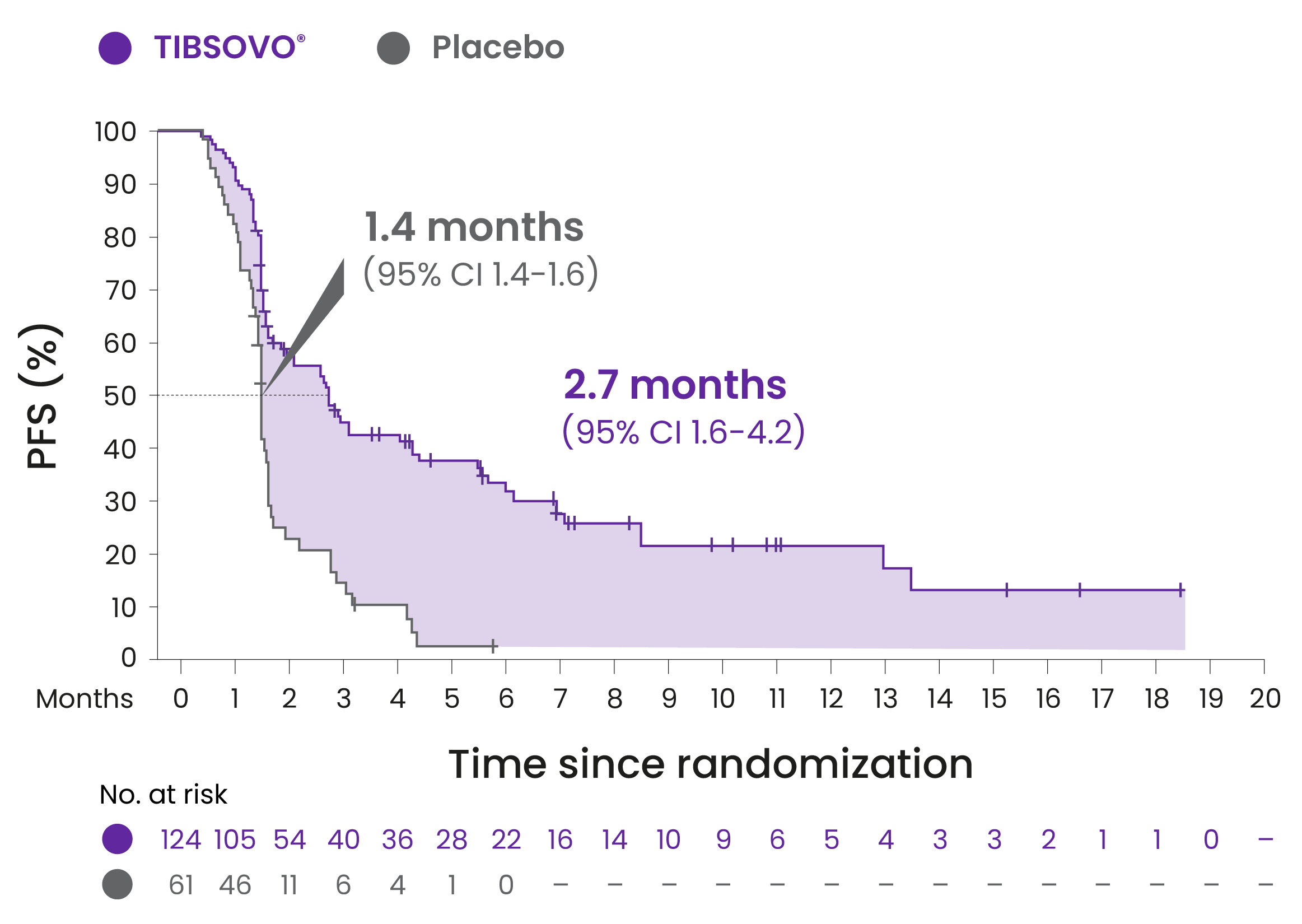
*PFS is defined as the time from randomization to progression or death from any cause.
Adapted from the TIBSOVO® Product Monograph.
Consult the TIBSOVO® Product Monograph
2-HG: 2-hydroxyglutarate; α-KG: alpha-ketoglutarate; AML: acute myeloid leukemia; AZA: azacitidine; PBO: placebo; CCA: cholangiocarcinoma; CI: confidence interval; CR: complete response; CRh: complete response with partial hematologic recovery; CTCAE: Common Terminology Criteria for Adverse Events; DLCO: diffusing capacity for carbon monoxide; ECG: electrocardiogram; ECOG PS: Eastern Cooperative Oncology Group Performance Status; EFS: event-free survival; eGFR: estimated glomerular filtration rate; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; IDH1: isocitrate dehydrogenase-1; IRC: Independent Radiology Centre; IV: intravenous; mIDH1: mutated isocitrate dehydrogenase-1; MOA: mechanism of action; NCCN: National Comprehensive Cancer Network; OR: odds ratio; OS: overall survival; PBO: placebo; PCR: polymerase chain reaction; PD: pharmacodynamics; PK: pharmacokinetics; QD: daily; RECIST: Response Evaluation Criteria In Solid Tumors; SC: subcutaneous.
References:
- TIBSOVO® Product Monograph. Servier Canada. July 19, 2024.
- Montesinos P, et al. N Engl J Med. 2022 Apr 21;386(16):1519–1531.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 2.2025 — January 27, 2025.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Duct Cancers Version 6.2024 — January 10, 2025.
- Abou-Alfa GK, et al. Lancet Oncol. 2020 Jun;21(6):796-807.
- Zhu AX, et al. JAMA Oncol. 2021 Nov 1;7(11):1669-1677.
- Dammacco F, Silvestris F, eds. Oncogenomics: From Basic Research to Precision Medicine. Academic Press; 2019.
Stay Connected with Servier Canada
Stay up to date on disease information, treatment options, our products, and patient support programs.